MP BOARD 2026 – Class 12 Chemistry Important Questions and Answers
This post contains important questions from the Board of Secondary Education 2026 for Class 12 Chemistry. These important questions have also been asked in previous years’ exams. At the end of this post, you will also be able to solve objective-type self-tests useful for the 2026 exam.

MP BOARD 2026- Objective Question Based Self Test – Chemistry Class 12
Question 1 : Define mole fraction.
Answer: The ratio of the number of moles of any one component (solute/solvent) present in a solution to the total number of moles present in the solution is called the mole fraction.
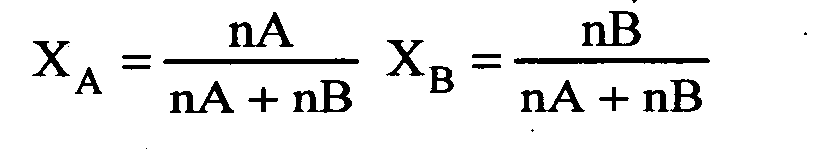
Question 2 : Define a solution.
Answer: A solution is a homogeneous mixture of two or more pure substances whose composition can be changed to a certain extent.
Question 3 : Write Faraday’s first law of electrolysis.
Answer: Faraday’s first law of electrolysis: The amount of substance liberated at an electrode during electrolysis is proportional to the amount of electricity passed through it.
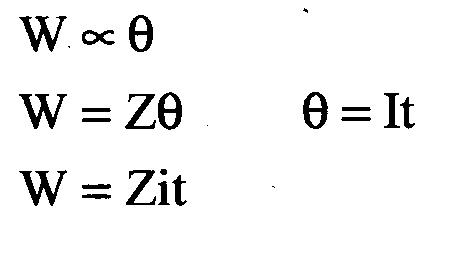
Question 4 : Write two functions of a salt bridge.
Answer: i. It connects two half-cells.
ii. It maintains electrical neutrality.
Question 5 : Write any two differences between the molecularity of a reaction and the order of a reaction.
Answer:
| Molecularity of the Reaction | Order of Reaction |
| i. This is a theoretical term | i. This is an experimental term |
| ii. It does not have a valid zero | ii. It can have a value of zero |
Question 6 : Write any two differences between the rate and constant of reaction.
Answer: Please write your own answers. (They will be uploaded to the site as soon as they become available.)
Question 7- Write the IUPAC names of the following coordination compounds.

Answer:
Question 8 : Write two uses of carboxylic acids.
Answer: Uses of Carboxylic Acids
i. Methanoic acid is used in the rubber, textile dyeing, leather, and electroplating industries.
ii. Acetic acid is used in food as vinegar.
Question 9 : Write only the chemical equation for the following change.
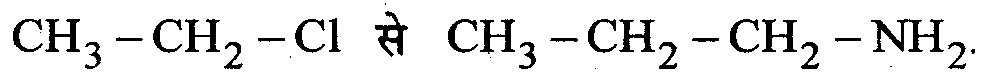
Answer:
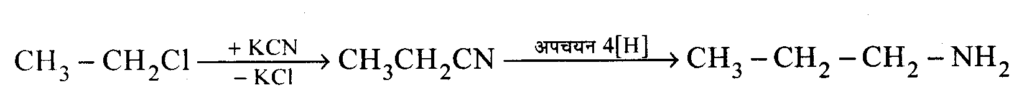
Question10 : Ethyl amine is more basic than ammonia. Give reasons.
Answer: Ethyl amine contains an ethyl group whose +I valence effect increases the availability of the lone electron on the nitrogen atom, which increases the basic nature of ethyl amine, whereas ammonia does not contain such a group.
Question 11- Write the following reaction with chemical equation
cannizzaro reaction
rojan mund reaction
Answer: Please write your own answers. (They will be uploaded to the site as soon as they become available.)
Question12 : Define and write the following: i. Rate-determining term; ii. Order of reaction.
Answer: i. Rate-determining term: The slowest step in a reaction is called the rate-determining term.
ii. Order of reaction: The order of reaction is the sum of all the powers that must be placed on the concentration terms in the rate equation to indicate the rate of the reaction.
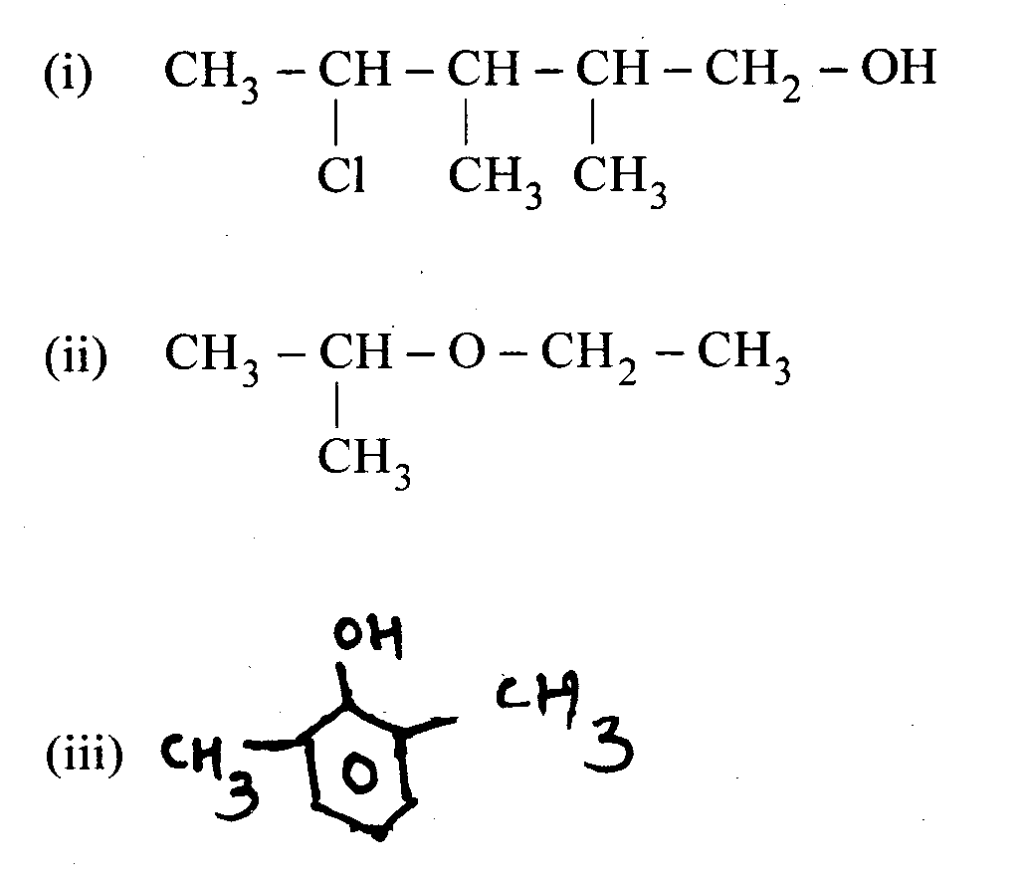
Question13 : Write the structure of the following compounds.
i. 4 chloro 2, 3 dimethyl pentane 1 ol ii. 2 ethoxy propane iii. 2, 6 dimethyl phenol
Answer:
Question14 : How would you obtain the following from CH3COOH? Write the chemical equations.
i. Acetic anhydride, ii. Ethyl acetate, iii. Acetyl chloride, iv. Acetamide, v. Ethyl alcohol.
Answer:
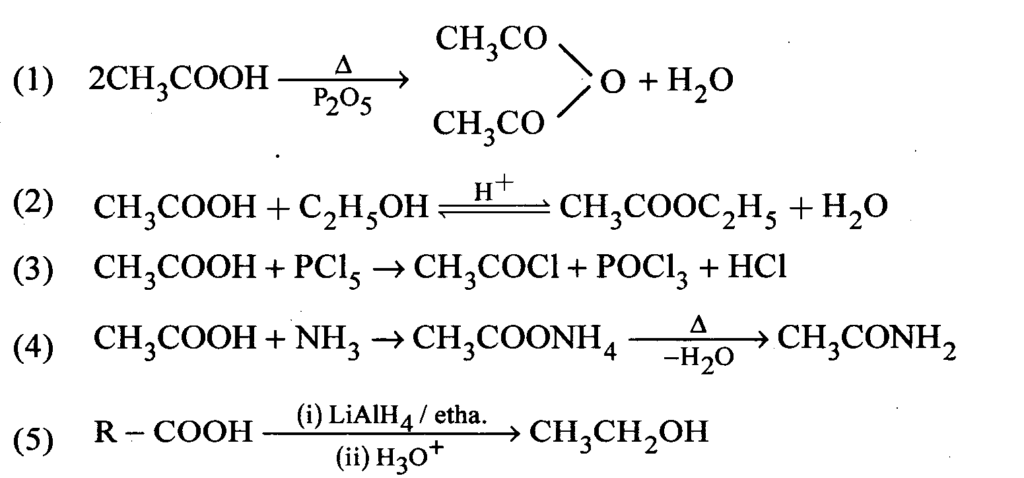
Question15 : Complete and write the following reactions.
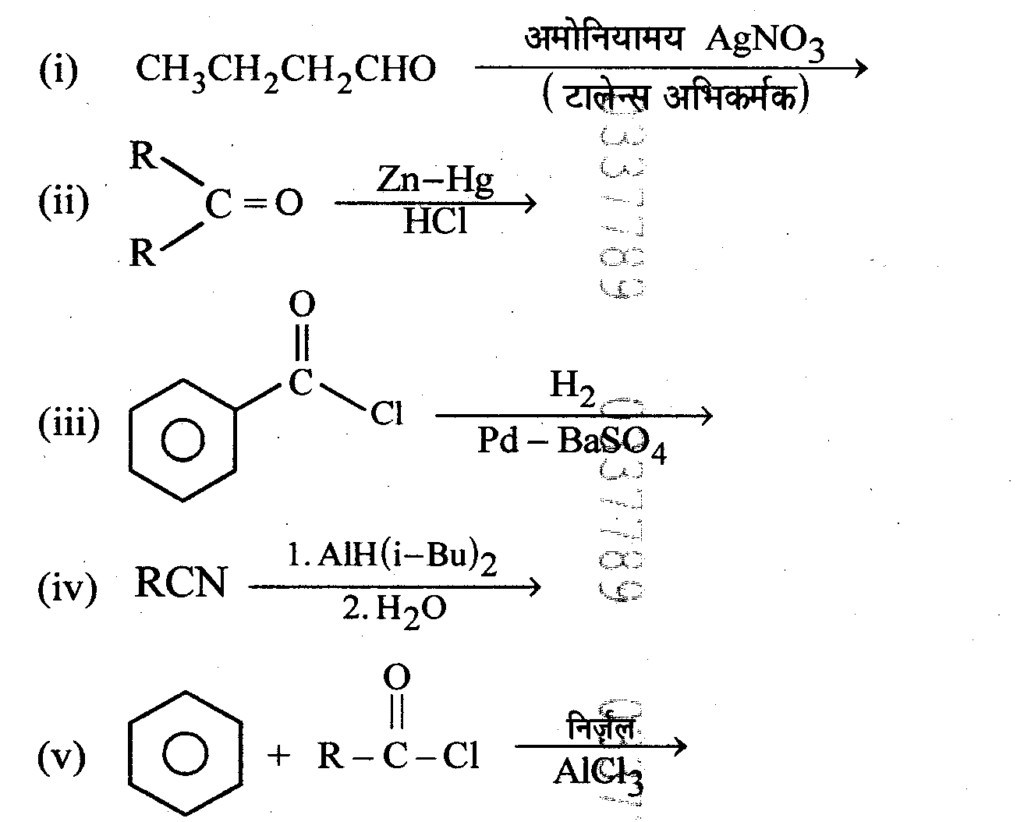
Answer:
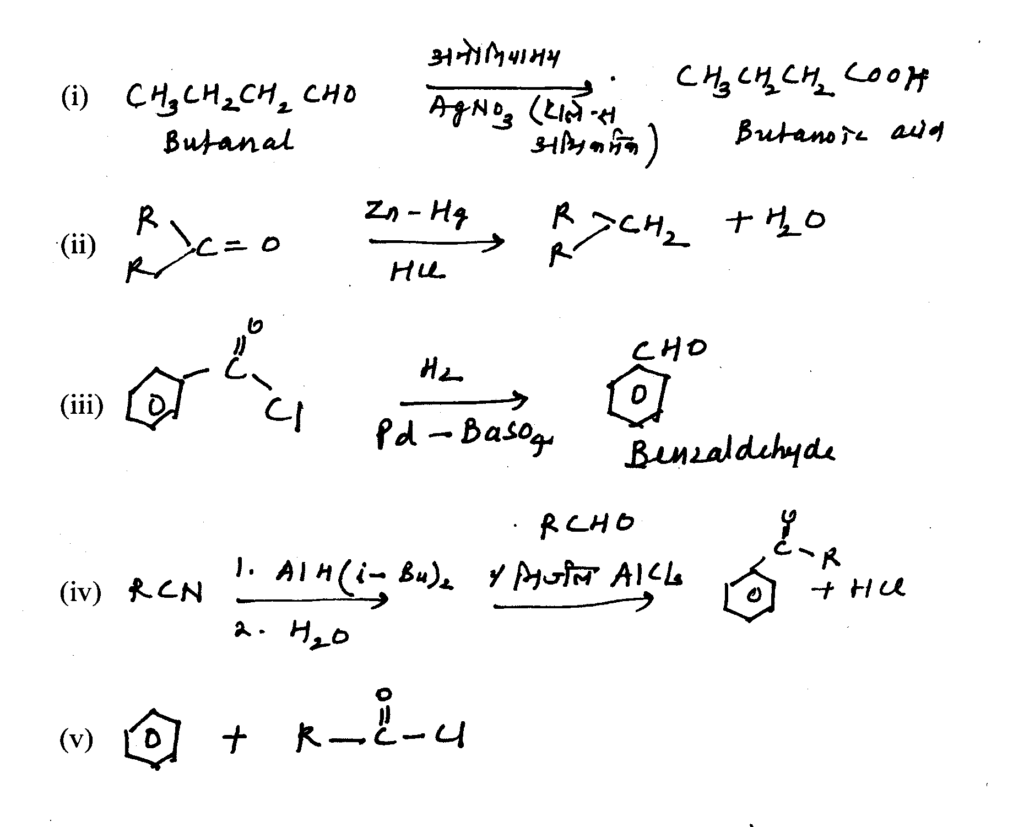
Question16 : Write the IUPAC formulas of the following coordination compounds.
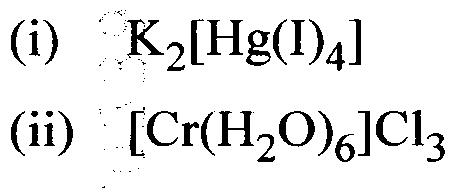
Answer:
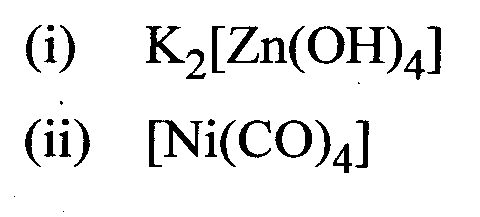
Question17 : Write Kohlrausch’s law and any two of its applications.
Answer: Please write your own answers. (They will be uploaded to the site as soon as they become available.)
Question18 : Now define the dip in freezing point and on its basis derive a mathematical expression for empirical determination of the solution.
Answer: Please write your own answers. (They will be uploaded to the site as soon as they become available.)
Question19 : Define osmotic pressure and on its basis derive a mathematical expression for empirical determination of solution.
Answer: Please write your own answers. (They will be uploaded to the site as soon as they become available.)
Question20 : Write the definition of enzyme with examples.
Answer: Please write your own answers. (They will be uploaded to the site as soon as they become available.)
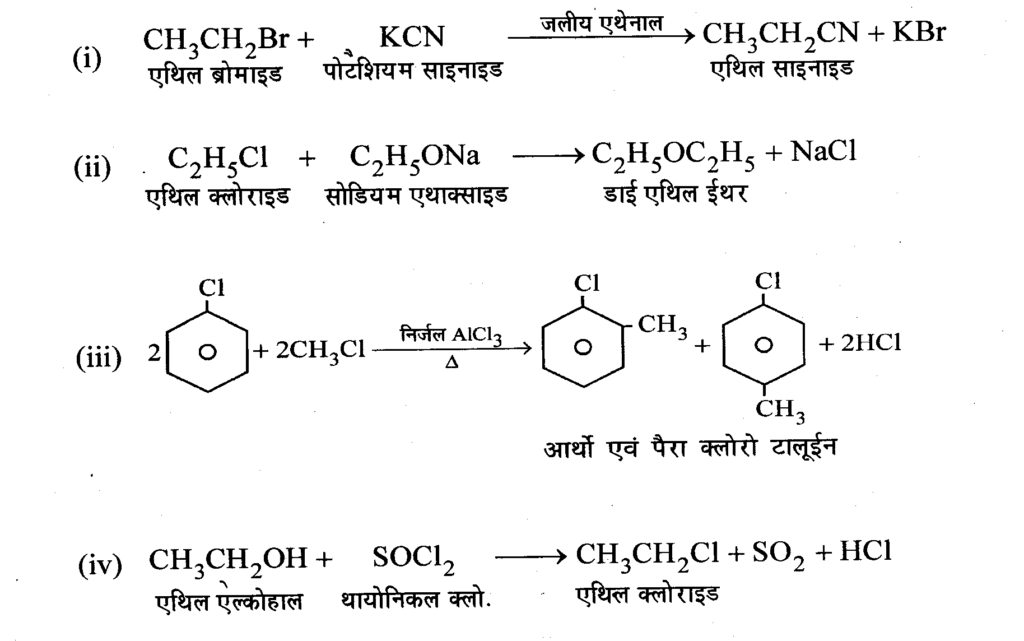
Question 21 : Complete the following reactions.
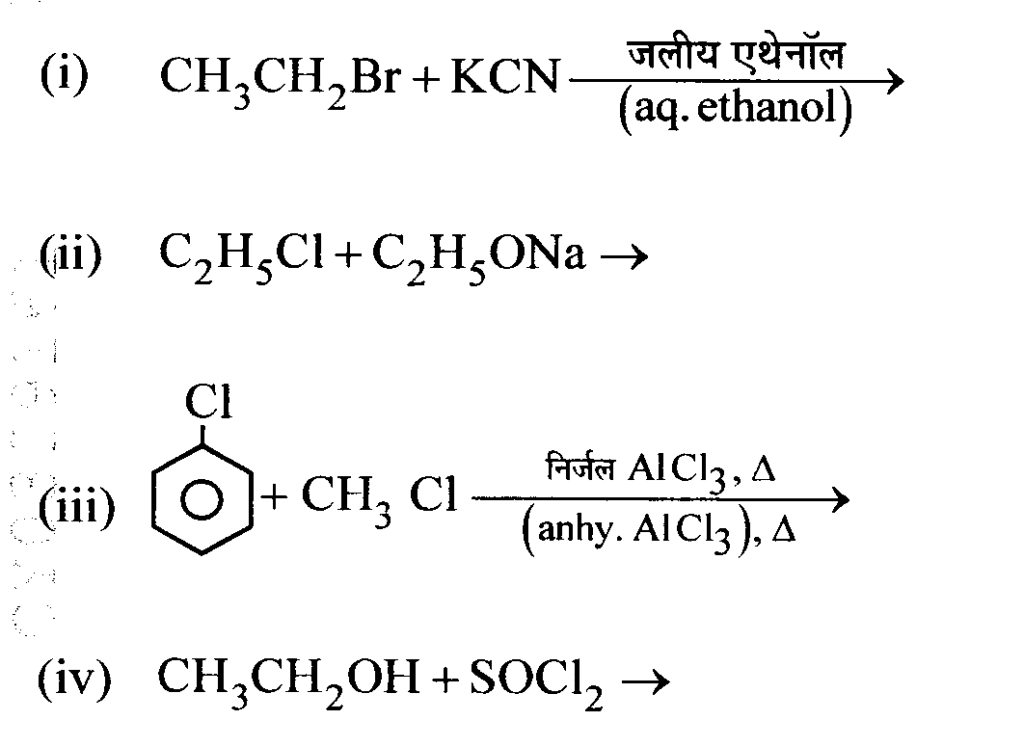
Answer:
You may also find this useful –
MP BOARD 2026- Objective Question Based Self Test – Chemistry Class 12
